Abstract
Background: Chemotherapy resistance and tumor immune microenvironment are the dual reasons for the poor efficacy of AML. Can we find a way to solve the two puzzles at one time? The leukemia cell membrane (LCM) coated-nanoparticle has been proved a cargo to precisely delivery drugs towards tumor cells by homologous targeting effect. Mn2+ can activate stimulator of interferon genes (STING) pathway to enhance the antitumor immune response. Therefore, we developed an LCM based hollow MnO2 nano-carrier (HM) encapsulated with antimitotic agent doxorubicin (DOX) (noted as LHMD) to achieve the targeted eliminating AML cells by virtue of MnO2 nanostructure-based immune- and chemotherapy synergy to improve the therapeutic effect.
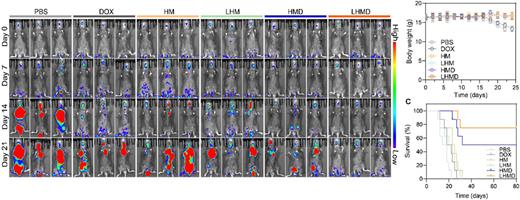
Methods: LHMD nanomedicine was designed by coating AML cell membrane onto DOX-loaded hollow MnO2 nano-carrier. The cell viability, cell injury behavior, homologous targeting effect and STING-mediated immune activation after LHMD nanomedicine treatment were investigated in vitro. In vivo BM homing properties and anti-AML efficacy were also analyzed in the AML mice model by intravenously injecting C1498 cells in C57BL/6J mice. After various treatments, C1498 proliferation in mice was observed by in vivo imaging system (IVIS). Mice survival curves and the body index, including body weight, blood biochemistry and blood routine were also recorded. After that, STING pathway activation and the resulted regulation of immune cell proportions and cytokine levels were also analyzed.
Results: HMnO2 nano-carrier with or without mC1498 coating (denoted as LHM and HM respectively) were obtained, and with DOX loading, LHMD nanomedicine could keep a fine dispersibility without any aggregation over time. LHMD treatment displayed the strongest the rate of cell death at the same DOX concentration, compared with free DOX and HMD treatments, LHMD treated group showed 51.8% and 56.1% enhancement in cytotoxicity at the effective DOX concentration of 59.75 μg mL-1. By evaluating the ability of activation STING via MnO2 nano-carrier, western blot assay confirmed the up-regulation of STING pathway-related proteins in LHM and HM treated groups, such as STING, IRF3, cGAS and type I interferon of IFN-β, proving that LHMD nanomedicine displayed the superior potential for STING pathway activation. In animal models, LHMD have shown promising anti-tumor efficacy and was observed accumulation in BM. The overall survival of PBS, DOX, HM, and LHM was 22 days, 27 days, 32 days, and 30 days respectively, the mice after LHMD treatment were survived more than 80 days, revealing the satisfactory effect of LHMD to prolong the survival time of leukemic mice. Compared with PBS group, the percentage of Th1 cells was up-regulated almost 1.65-fold after LHMD treatment after LHMD treatment, the percentages of Th2 were down-regulated while Th17 was up-regulated after LHMD administration, the ratio of Th1/Th2 was increased in LHMD-treated group, manifesting the immune enhancing efficacy with LHMD.
Conclusion: We developed a bio-inspired nanomedicine (LHMD) to specifically target in the bone marrow (BM) and deliver chemotherapeutic agents for potent immune therapy and chemotherapy against AML. Such nanomedicine can trigger the STING pathway activation, and the released Mn2+ could improve MRI signal for AML detection and BM-specific imaging. LHMD could precisely target leukemic cells in BM, overcome chemoresistance, and prolong the overall survival time of mice simultaneously. The combination of chemo- and immunotherapy approach induce the effective anti-tumor immune responses and inhibition of AML growth. Such a bio-inspired strategy for BM-targeted delivery might be a potent challenge to design more effective and accurate platform in current blood cancers.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal